
Fassett Farm Nursery in Jaffrey focuses on native plants
Fassett Farm Nursery in Jaffrey, which has a focus on growing and selling native plants, is back for its second season starting May 4 with a new storefront, expanded hours and new consultation offerings.The nursery is owned by Aaron Abitz, and run on...

UPDATE: Drivers identified in Jaffrey dump truck crash
A collision between a sedan and a dump truck on Route 202 in Jaffrey Wednesday afternoon caused a significant fuel spill across the highway and led to one driver being taken to the hospital.According to police reports, a 2023 Nissan Rogue, driven by...
Most Read
 UPDATE: Drivers identified in Jaffrey dump truck crash
UPDATE: Drivers identified in Jaffrey dump truck crash
 Conant baseball shows its strength in win over Mascenic
Conant baseball shows its strength in win over Mascenic
 Group looks to close divide in Mascenic district
Group looks to close divide in Mascenic district
 Bernie Watson of Bernie & Louise dies at 80
Bernie Watson of Bernie & Louise dies at 80
 Rindge Recreation Department organizes a trip to Converse Meadow
Rindge Recreation Department organizes a trip to Converse Meadow
 Scott Bakula starring in Peterborough Players’ ‘Man of La Mancha’
Scott Bakula starring in Peterborough Players’ ‘Man of La Mancha’
Editors Picks
 HOUSE AND HOME: The Old Parsonage in Antrim is a ‘happy house’
HOUSE AND HOME: The Old Parsonage in Antrim is a ‘happy house’
 Former home of The Folkway in Peterborough is on the market
Former home of The Folkway in Peterborough is on the market
 Two Jaffrey-Rindge Destination Imagination teams move on to Globals
Two Jaffrey-Rindge Destination Imagination teams move on to Globals
 Old Homestead Farm in New Ipswich requests variance for short-term rental cabins
Old Homestead Farm in New Ipswich requests variance for short-term rental cabins
Sports

Katalina Davis dazzles in Mascenic shutout win
The Mascenic softball team soared to a 7-0 shutout win over regional and interdivisional rival Conant thanks to a dazzling performance on both sides of the ball from senior pitcher Katalina Davis. “When Kat’s on, they’re not hitting, so she was...
 LOCAL SPORTS ROUNDUP: Conant girls’ tennis gets first win
LOCAL SPORTS ROUNDUP: Conant girls’ tennis gets first win
 Conant girls’ tennis continues to seek improvement
Conant girls’ tennis continues to seek improvement
 Jack Kidd and Kidd Gloves make donation to Mt. Monadnock Little League
Jack Kidd and Kidd Gloves make donation to Mt. Monadnock Little League
Opinion

Opinion: It’s time to properly fund education
Susan McKevitt lives in Bradford. On April 11, the New York Times ran an article entitled: “It’s Time to End the Quiet Cruelty of Property Taxes.” Like many towns, Bradford recently had its town meeting, and we experienced this cruelty.Over 200...
Business
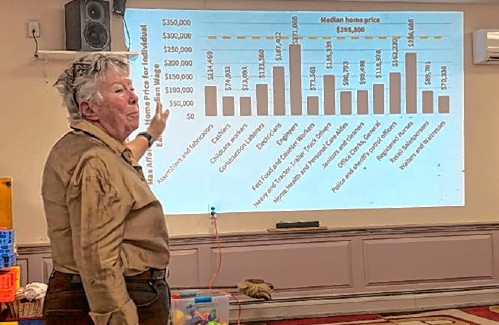
BUSINESS QUARTERLY: Grants allow towns to study housing
In 2022 the State of New Hampshire, in recognition of the housing shortage in the state, created a grant program to fund work in towns that wished to explore the housing situation in their town and consider ways to increase housing availability and...
 BUSINESS QUARTERLY: Dan Petrone – A guide to the commission settlement
BUSINESS QUARTERLY: Dan Petrone – A guide to the commission settlement
 BUSINESS QUARTERLY – New housing projects could provide relief
BUSINESS QUARTERLY – New housing projects could provide relief
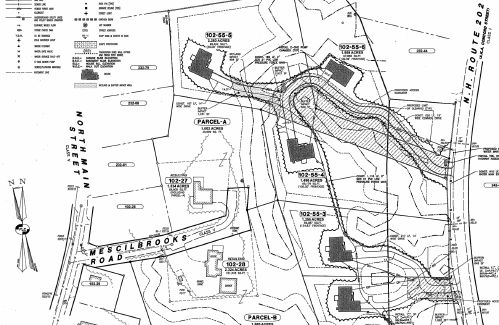 BUSINESS QUARTERLY – Antrim Planning Board approves Battaglia subdivision
BUSINESS QUARTERLY – Antrim Planning Board approves Battaglia subdivision
Arts & Life
Greenfield Roadside Roundup is April 27
The Greenfield Conservation Commission will hold its annual Roadside Roundup in honor of Earth Day Saturday, April 27, rain or shine.People can get their blue bags from the Recycling Center, Stephenson Memorial Library, Greenfield Post Office or...
 Bernie Watson of Bernie & Louise dies at 80
Bernie Watson of Bernie & Louise dies at 80
 Cosy Sheridan speaks and performs for Monadnock Writers’ Group
Cosy Sheridan speaks and performs for Monadnock Writers’ Group
 ‘The Last Laugh’ coming to Town Hall Theatre in Wilton
‘The Last Laugh’ coming to Town Hall Theatre in Wilton
 Echoes of Floyd performs Saturday at Peterborough Town House
Echoes of Floyd performs Saturday at Peterborough Town House
Obituaries
 Elizabeth "Betty" G. Mahon
Elizabeth "Betty" G. Mahon
Boscawen, NH - Elizabeth "Betty" G. Mahon, age 93, passed away peacefully on Saturday, April 13, 2024 with family by her side. She was born in Salem, MA daughter of the late John and Elizabeth (Tansey) Gannon. She was predeceased by he... remainder of obit for Elizabeth "Betty" G. Mahon
 Phillip A. Avery
Phillip A. Avery
Bennington NH - Phillip A Avery, 81, of Bennington, peacefully passed away on April 4,2024 after a long and hard-fought battle with cancer, while under hospice care at Monadnock Community Hospital with his wife Ann and the love of his f... remainder of obit for Phillip A. Avery
 Kevvin W. Sawtelle 31
Kevvin W. Sawtelle 31
Kevvin W. Sawtelle, 31 Jaffrey, NH - Kevvin W. Sawtelle, 31, of Rindge, died peacefully on April 5, 2024, in the arms of his family at the Dartmouth-Hitchcock Medical Center in Lebanon, NH after a long battle with brain cancer. Kevvin w... remainder of obit for Kevvin W. Sawtelle 31
 Constance Boldini
Constance Boldini
Westmoreland, NH - Constance Marie (Wilson) Boldini, died April 16, 2024. She is predeceased by her husband Guido Boldini and her son Peter Vaillancourt. She is survived by two daughters, Anne Young of Tennessee, Brenda Bryer of Stoddar... remainder of obit for Constance Boldini

 T & W Handyman Services in Jaffrey celebrates new location
T & W Handyman Services in Jaffrey celebrates new location
 BACKYARD NATURALIST: Phil Brown – Kestrel conservation expands in the Contoocook Valley
BACKYARD NATURALIST: Phil Brown – Kestrel conservation expands in the Contoocook Valley
 Greenfield Community Power Committee approves identifying possible suppliers
Greenfield Community Power Committee approves identifying possible suppliers
 Wilton Select Board discusses options for ARPA funds
Wilton Select Board discusses options for ARPA funds
 The Rev. Barbara Thorngren of Temple works to build connections
The Rev. Barbara Thorngren of Temple works to build connections

 HIGH SCHOOL SPORTS ROUNDUP: Tasha MacNeil leads the way for ConVal at Pelham Invita
HIGH SCHOOL SPORTS ROUNDUP: Tasha MacNeil leads the way for ConVal at Pelham Invita
